-
PDF
- Split View
-
Views
-
Cite
Cite
Jonathan Maurice Chemouny, Margarita Hurtado-Nedelec, Héloïse Flament, Sanae Ben Mkaddem, Eric Daugas, François Vrtovsnik, Laureline Berthelot, Renato C. Monteiro, Protective role of mouse IgG1 in cryoglobulinaemia; insights from an animal model and relevance to human pathology, Nephrology Dialysis Transplantation, Volume 31, Issue 8, August 2016, Pages 1235–1242, https://doi.org/10.1093/ndt/gfv335
Close - Share Icon Share
Abstract
Strait et al. described a novel mouse model of cryoglobulinaemia by challenging mice deficient in the immunoglobulin (Ig)G1 subclass (γ1− mice) with goat anti-mouse IgD [5]. The phenotype of wild-type mice was not remarkable, whereas γ1− mice developed IgG3 anti-goat IgG cryoglobulins as well as severe and lethal glomerulonephritis. Renal phenotype could not be rescued in γ1− mice by the deletion of C3, fragment crystalline γ receptor (FcγR) or J chain. On the other hand, early injection of IgG1, IgG2a or IgG2b inhibited the pathogenic effects of IgG3 in an antigen-dependent manner even in the absence of the FcγRIIb, an anti-inflammatory receptor. The authors concluded that the pathogenic role of IgG3 and the protective characteristic of IgG1 in this model were not explained by their abilities to bind to FcRs or effector molecules but are rather due to structural discrepancies enhancing the precipitation properties/solubility of IgG3/IgG1-containing immune complexes. The present article aims to discuss the current knowledge on IgG biology and the properties of IgGs explaining their differential propensity to acquire cryoglobulin activity.
INTRODUCTION
Cryoglobulinaemia is defined as the presence in serum of immunoglobulins (Igs) that can precipitate at a temperature lower than 37°C and can be redissolved upon rewarming. In 1974, Brouet et al. [1] set up a classification of cryoglobulins which is still in use today. They distinguished three types of cryoglobulins based on the protein components of the precipitates. Type I cryoglobulins are monoclonal Igs, usually IgM or IgG. Type II and Type III cryoglobulins, called mixed cryoglobulins, are composed of two or more Igs. They are composed of a polyclonal Ig and a second Ig (usually IgM or IgG), which can be either monoclonal (Type II) or polyclonal (Type III). Cryoglobulins may be found in the contexts of B-cell lymphoproliferative diseases, infections (or lymphoproliferative disorders occurring in the course of hepatitis C virus (HCV) infections, which account for most cases of mixed cryoglobulins [2]) or autoimmune diseases. In the absence of associated conditions, they are deemed as primary.
Cryoglobulins may be responsible for a systemic vasculitis called cryoglobulinaemic vasculitis, which may include general symptoms and involve skin, joints, kidneys and nervous system (peripheral more frequently than central). The severity of the clinical manifestations is highly variable. They may be limited to purpuric skin lesions or generate life-threatening conditions [3]. Moreover, cryoglobulinaemia does not systematically lead to clinical manifestations. Indeed, in a recent study led in Origgio, Italy, 34 out of 147 randomly selected subjects were tested positive for circulating cryoglobulins, of whom only 4 were identified as having cryoglobulinaemic vasculitis [4]. The reasons underlying these discrepancies are not fully understood. In a recent letter to the journal Nature, Strait et al. [5] lifted a corner of the veil by unravelling a receptor- and complement-independent IgG-mediated pathogenic mechanism in the context of murine cryoglobulinaemia. The present review aims to expose the current knowledge on IgGs and their implications in the pathogenicity of cryoglobulins in the light of the results of Strait et al. [5]. Consequently, although the very large majority of cryoglobulinaemia cases involve an IgM component, and the B-cell biology is impaired in this condition, neither IgM biology nor Ig production will be discussed.
STRUCTURE OF IgG
Common features of IgG
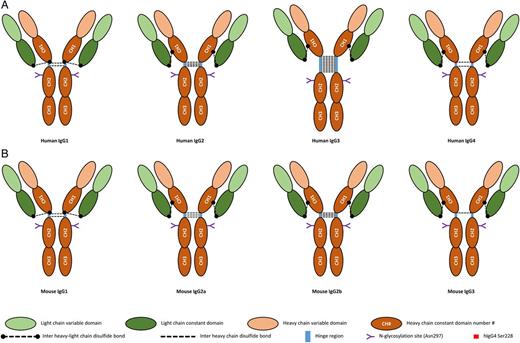
IgG is the most abundant Ig class in serum. With regard to all antibodies, IgGs, like any antibody, are produced by B-cells after maturation into plasma cells upon stimulation by a cognate antigen. The general structure of IgGs is similar to that of the other Igs and consists in the association of two heavy chains (HCs) and two light chains (LCs) (Figure 1A), these latter being either kappa or lambda. The IgG heavy chain is composed of four domains: the variable region (VH) at the amino-terminal end of the chain and three constant domains (CH1, CH2 and CH3) numbered from the amino-terminal to the carboxy-terminal end of the protein. HCs are linked to the LCs and to one another by disulphide bonds. The cysteines responsible for the disulphide bonds between the HCs are located in a region between the CH1 and CH2 domains, named the hinge region as it is supporting the flexibility between the Fab (fragment antigen binding, composed of one CH1, one VH and one LC) and the Fc (fragment crystalline, composed of two CH2 and CH3).
Structure of human (A) and mouse (B) IgG. The inter-heavy–light-chain disulphide bonds are represented as in the primary structure of IgG. The folding of the proteins brings the cysteine involved in these bonds into the vicinity of the hinge region in all IgGs.
IgG subtypes
In humans, differences in the amino-acid (aa) sequences of the constant domains of IgGs discriminate four IgG subtypes numbered according to their relative abundance in serum as IgG1, IgG2, IgG3 and IgG4 [6]. The differences between these four subtypes are principally found in the hinge region and the CH2 domain. Indeed, both of those sites are functionally important since the flexibility of the antibody, supported by the hinge region, determines the capacity of the antibody to bind to epitopes far from one another and since the Ig effector functions are mediated through the binding of effector molecules or receptors to the CH2 domain.
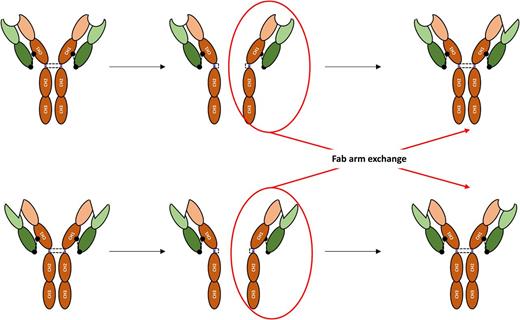
IgG1 is the most abundant IgG subclass in the serum; its hinge region contains 15 aa and possesses 2 disulphide bonds linking the HCs. IgG2 and IgG4 have a 12 aa-long hinge region. The flexibility of the IgG2 hinge region is further reduced by the presence of four disulphide bonds linking the HC. IgG4 has the same amount of disulphide bonds as IgG1, but a serine residue at position 228 confers more flexibility to the region and allows intra-chain disulphide bonds between the cysteines at positions 226 and 229, resulting in the cleavage of two half IgG4 molecules each containing an HC and an LC. These half molecules may then combine to another half molecule produced by another B-cell clone with a different antigen specificity resulting in a bispecific antibody, a mechanism referred to as the IgG4 Fab arm exchange (Figure 2). A direct consequence is the lesser propensity for these bispecific IgG4 to form immune complexes if an antigen does not present both recognized epitopes [7]. IgG3 possesses the longest hinge region among IgGs, comprising up to 62 aa (Figure 1A). The distance between the Fab and the Fc confers to IgG3 a more important flexibility, enhancing the capacity of this antibody subclass to bind two epitopes in a broader range of spatial configuration.
IgG4 Fab arm exchange and formation of bispecific IgG4. Key to domains as in Figure 1.
IgG glycosylation
IgGs are glycosylated in the CH2 domain [8], near the hinge region, where a highly conserved glycosylation site has been described. The asparagine present in the CH2 domain of all IgGs (hereafter referred to as Asn297) bears a biantennary-type oligosaccharide side chain whose core structure is composed of seven monosaccharides: four N-acetylglucosamines and three mannoses. Supplemental monosaccharides may be added to this core structure, such as galactose, sialic acid, additional N-acetylglucosamines and fucoses. The different glycoforms of IgGs may impact their functions by modulating their affinity to Fc receptors (FcRs) through different mechanisms. The conserved oligosaccharide is responsible for maintaining the three-dimensional structure of IgG-Fc, allowing their interactions with FcR [9]. Moreover, the composition of these oligosaccharides is also able to modulate the affinity of IgGs to their receptors. Indeed, both sialic acid [10] and fucose [11] were shown to decrease this affinity. On the other hand, intravenous Ig enriched for sialic acid were shown to have increased immunomodulatory properties [10], through an increased affinity towards a specific C-type lectin, SIGN-R1, in mouse (equivalent to human DC-SIGN) [12]. Finally, the different IgG glycoforms may be able to activate the complement through the lectin pathways [13] and to bind non-conventional IgG receptors with affinity towards sugar moieties [14] such as the aforementioned DC-SIGN/SIGN-R1.
IgG FUNCTIONS
Antigen binding is the only function equally provided by all IgG subtypes although not all of them are able to form immune complexes for reasons already exposed. Indeed only the variable domains and therefore the V(D)J rearrangement and affinity maturation mechanisms generating the complementarity-determining regions would be involved. Conversely, distinction between IgG subtypes is supported by differences in the constant domains resulting in different interactions with receptors or effector molecules.
C1q and complement activation
Upon binding to their antigen, IgG1 and IgG3 (and IgM) form immune complexes that activate the complement cascade through the classical pathway initiated by the C1q molecule. This binding induces an auto-activation of C1q, which acquires a protease activity through its association with C1r and C1s and cleaves C4 and C2. The C4b and C2a fragments of these proteins associate to form the C3 convertase that cleaves native C3, the central protein of complement. Complement activation leads to (i) cell lysis through the membrane attack complex, (ii) inflammation through the generation of anaphylatoxins and chemoattractant, and (iii) immune complex clearance. Therefore, complement activation by immune complexes is a key event to the response against pathogens (Table 1). It has also been suggested that some IgG glycoforms were able to activate complement through the lectin [15] and the alternative [16] pathways.
Affinity of human and mouse IgGs to their receptor and C1q.
| . | Human . | Mouse . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgG1 . | IgG2 . | IgG3 . | IgG4 . | Expression patterna . | IgG1 . | IgG2a . | IgG2b . | IgG3 . | Expression patterna . | |
| FcγRI | +++ | − | +++ | +++ | Monocytes/macrophages and dendritic cells (neutrophils, mast cells) | − | +++ | − | − | Macrophages |
| FcγRIIAb | ++ | + | + | + | Myeloid cells | |||||
| FcγRIIB | + | ± | + | + | Circulating B-cells, basophils, macrophages and dendritic cells | ++ | + | ++ | − | Mast cells, basophils, B-cells |
| FcγRIICb | + | ± | + | + | NK cells, monocytes and neutrophils | |||||
| FcγRIIIA | ++ | ± | ++ | + | NK cells and monocytes/macrophages | + | + | + | − | Mast cells, basophils, macrophages, neutrophils, NK cells and B-cells |
| FcγRIIIBb | + | − | ++ | − | Neutrophils | |||||
| FcγRIVc | − | +++ | +++ | − | Macrophages and neutrophils | |||||
| FcγRn | +++ | +++ | ± | +++ | Epithelial cells, dendritic cells, monocytes/macrophages and neutrophils | +++ | +++ | +++ | ± | Epithelial cells, dendritic cells, monocytes/macrophages and neutrophils |
| FcRL4b | − | − | ++ | +++ | B-cells | |||||
| FcRL5 | ++ | +++ | ++ | ± | B-cells | ND | ND | ND | ND | B-cells |
| C1q binding | +++ | − | ++ | − | NA | ± | +++ | ++ | ++ | |
| . | Human . | Mouse . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgG1 . | IgG2 . | IgG3 . | IgG4 . | Expression patterna . | IgG1 . | IgG2a . | IgG2b . | IgG3 . | Expression patterna . | |
| FcγRI | +++ | − | +++ | +++ | Monocytes/macrophages and dendritic cells (neutrophils, mast cells) | − | +++ | − | − | Macrophages |
| FcγRIIAb | ++ | + | + | + | Myeloid cells | |||||
| FcγRIIB | + | ± | + | + | Circulating B-cells, basophils, macrophages and dendritic cells | ++ | + | ++ | − | Mast cells, basophils, B-cells |
| FcγRIICb | + | ± | + | + | NK cells, monocytes and neutrophils | |||||
| FcγRIIIA | ++ | ± | ++ | + | NK cells and monocytes/macrophages | + | + | + | − | Mast cells, basophils, macrophages, neutrophils, NK cells and B-cells |
| FcγRIIIBb | + | − | ++ | − | Neutrophils | |||||
| FcγRIVc | − | +++ | +++ | − | Macrophages and neutrophils | |||||
| FcγRn | +++ | +++ | ± | +++ | Epithelial cells, dendritic cells, monocytes/macrophages and neutrophils | +++ | +++ | +++ | ± | Epithelial cells, dendritic cells, monocytes/macrophages and neutrophils |
| FcRL4b | − | − | ++ | +++ | B-cells | |||||
| FcRL5 | ++ | +++ | ++ | ± | B-cells | ND | ND | ND | ND | B-cells |
| C1q binding | +++ | − | ++ | − | NA | ± | +++ | ++ | ++ | |
+++: Ka > 107; ++: Ka > 106; +: Ka > 105; ±: Ka > 104; −: no binding; Ka: affinity constant; NA: not applicable; ND: no data; NK: natural killer.
aConstitutive expression (inducible expression).
bThis receptor is not expressed in mouse.
cThis receptor is not expressed in humans.
Affinity of human and mouse IgGs to their receptor and C1q.
| . | Human . | Mouse . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgG1 . | IgG2 . | IgG3 . | IgG4 . | Expression patterna . | IgG1 . | IgG2a . | IgG2b . | IgG3 . | Expression patterna . | |
| FcγRI | +++ | − | +++ | +++ | Monocytes/macrophages and dendritic cells (neutrophils, mast cells) | − | +++ | − | − | Macrophages |
| FcγRIIAb | ++ | + | + | + | Myeloid cells | |||||
| FcγRIIB | + | ± | + | + | Circulating B-cells, basophils, macrophages and dendritic cells | ++ | + | ++ | − | Mast cells, basophils, B-cells |
| FcγRIICb | + | ± | + | + | NK cells, monocytes and neutrophils | |||||
| FcγRIIIA | ++ | ± | ++ | + | NK cells and monocytes/macrophages | + | + | + | − | Mast cells, basophils, macrophages, neutrophils, NK cells and B-cells |
| FcγRIIIBb | + | − | ++ | − | Neutrophils | |||||
| FcγRIVc | − | +++ | +++ | − | Macrophages and neutrophils | |||||
| FcγRn | +++ | +++ | ± | +++ | Epithelial cells, dendritic cells, monocytes/macrophages and neutrophils | +++ | +++ | +++ | ± | Epithelial cells, dendritic cells, monocytes/macrophages and neutrophils |
| FcRL4b | − | − | ++ | +++ | B-cells | |||||
| FcRL5 | ++ | +++ | ++ | ± | B-cells | ND | ND | ND | ND | B-cells |
| C1q binding | +++ | − | ++ | − | NA | ± | +++ | ++ | ++ | |
| . | Human . | Mouse . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgG1 . | IgG2 . | IgG3 . | IgG4 . | Expression patterna . | IgG1 . | IgG2a . | IgG2b . | IgG3 . | Expression patterna . | |
| FcγRI | +++ | − | +++ | +++ | Monocytes/macrophages and dendritic cells (neutrophils, mast cells) | − | +++ | − | − | Macrophages |
| FcγRIIAb | ++ | + | + | + | Myeloid cells | |||||
| FcγRIIB | + | ± | + | + | Circulating B-cells, basophils, macrophages and dendritic cells | ++ | + | ++ | − | Mast cells, basophils, B-cells |
| FcγRIICb | + | ± | + | + | NK cells, monocytes and neutrophils | |||||
| FcγRIIIA | ++ | ± | ++ | + | NK cells and monocytes/macrophages | + | + | + | − | Mast cells, basophils, macrophages, neutrophils, NK cells and B-cells |
| FcγRIIIBb | + | − | ++ | − | Neutrophils | |||||
| FcγRIVc | − | +++ | +++ | − | Macrophages and neutrophils | |||||
| FcγRn | +++ | +++ | ± | +++ | Epithelial cells, dendritic cells, monocytes/macrophages and neutrophils | +++ | +++ | +++ | ± | Epithelial cells, dendritic cells, monocytes/macrophages and neutrophils |
| FcRL4b | − | − | ++ | +++ | B-cells | |||||
| FcRL5 | ++ | +++ | ++ | ± | B-cells | ND | ND | ND | ND | B-cells |
| C1q binding | +++ | − | ++ | − | NA | ± | +++ | ++ | ++ | |
+++: Ka > 107; ++: Ka > 106; +: Ka > 105; ±: Ka > 104; −: no binding; Ka: affinity constant; NA: not applicable; ND: no data; NK: natural killer.
aConstitutive expression (inducible expression).
bThis receptor is not expressed in mouse.
cThis receptor is not expressed in humans.
IgG receptors
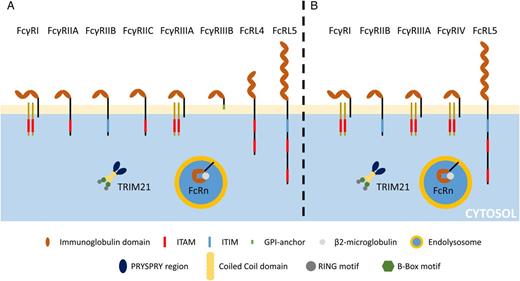
The canonical human IgG receptor family contains six members belonging to the Ig receptor superfamily (Figure 3). Out of the six canonical FcRs, four are activating receptors (FcγRI, FcγRIIA, FcγRIIC and FcγRIIIA), one is an inhibiting receptor (FcγRIIB) and the last one (FcγRIIIB) was not labelled because of the absence of signalling motif. Four additional receptors are described. Two of them are expressed at the cell membrane (FcRL4, FcRL5), the other two being intracellular (FcRn, TRIM21). These receptors are deemed as non-canonical because of their intracellular location (FcRn, TRIM21) or their affinity towards other Ig classes (FcRL4 and FcRL5).
FcγRI and FcγRIIIA are receptors with three and two extracellular domains, respectively, and composed of transmembrane and cytoplasmic domains that are non-covalently associated with a common γ chain, a homodimeric protein bearing the immunoreceptor tyrosine-based activation motif (ITAM). The two other activating receptors, the FcγRIIA and the FcγRIIC, are not associated with an FcγR and consist of single-chain transmembrane proteins bearing an ITAM-like motif on their intracytoplasmic tail. Although the ITAM confers upon these four receptors the ability to activate cells, we showed that both FcγRIIA [17] and FcγRIIIA [18] may exert immunomodulatory function upon monovalent or divalent binding. The FcγRIIB is the only canonical IgG receptor bearing an immunoreceptor tyrosine-based inhibition motif (ITIM). Similarly to the the FcγRIIA, the FcγRIIB is composed of one single transmembrane protein bearing the signalling motif on its intracellular tail [19].
FcγRIIIB is a glycosylphosphatidylinositol-anchored receptor, which is expressed on neutrophils and may be detected on basophils. In spite of its lack of intracellular signalling domain, it is able to facilitate FcγRIIA activity through calcium mobilization [20].
The neonatal Fc receptor (FcRn) is a heterodimeric receptor related to Type I major histocompatibility complex protein composed of a transmembrane heavy α-chain containing three extracellular domains associated with the β2-microglobulin LC. FcRn is found in the endolysosomal system where the acidic environment modifies the spatial conformation of IgG, unravelling binding sites to FcRn. FcRn is expressed by epithelial cells, such as podocytes [21], where it is responsible for IgG transcytosis, and in dendritic cells (DCs), monocytes/macrophages and neutrophils where it plays a significant role in IgG recycling and extended half-life and cross-presentation [22]. The IgG binding site to the FcRn is different from the one to conventional FcRs; it is located at the CH2–CH3 interface [23].
Fc receptor-like 4 and 5 (FcRL4, FcRL5) recently proved to be bona fide FcRs [24], since FcRL4 binds human IgG3 (hIgG3) and hIgG4 and FcRL5 binds hIgG1, hIgG2, hIgG3 and hIgG4. These receptors are expressed by B-cells. Both of them contain ITIM and exert inhibitory effects towards B-cell receptor activation by cognate antigens [25] although FcRL5 also contains one potential ITAM [26].
Tripartite-motif protein 21 (TRIM21) is a recently described, ubiquitous intracellular homodimeric FcR able to bind antibodies present on the surface of intracellular pathogens. This receptor may play a critical role in antiviral defences [27]. Similarly to FcRn, Trim21 interacts with epitopes in both the CH2 and CH3 domains of IgG Fc (Table 1).
MOUSE AND HUMAN IgG: DIFFERENCES AND SIMILARITIES
As most experimental data, including those that are highlighted in the present review, were obtained in mice, the similarity and differences with regard to the murine and the human IgG system should be kept in mind.
Mice also have four IgG subtypes (IgG1, IgG2a, IgG2b and IgG3), which share 53 to 69% homology only (compared with 80 to 92% in humans). The general structure of murine IgG (mIgG) is pretty similar to the one of human IgG with each antibody consisting of two LCs and two HCs composed of two and four domains, respectively. As in humans, the CH2 domain contains a conserved N-linked glycosylation site with the same sugar chains identified as in humans [28] (Figure 1B).
The mIgG hinge regions are composed of 13 and 22 aa, which makes the hIgG3 hinge region (62 aa) longer than the murine. In addition, the lengths of mIgG2a and mIgG3 (16 aa) hinge regions are pretty close to that of hIgG1 (15 aa). Similarly, mIgG1 has only one more aa (13 aa) in its hinge region than hIgG2 and hIgG4 (12 aa). In accordance, the hIgG3 hinge region is the one with the most flexibility (hIgG3 > mIgG2b > mIgG2a > hIgG1 > mIgG3 > mIgG1 > hIgG4 > hIgG2). The lower flexibility of mIgG3 compared with mIgG2 and hIgG1, in spite of the hinge region being similar in length or longer, may be explained by the presence of a polyproline helix in the upper hinge [29].
The discrepancies between hIgG and mIgG extend to IgG receptors. Mice express four IgG receptors: the activating FcγRI, FcγRIII and FcγRIV, which are absent in humans [30], and the inhibitory FcγRIIB. Human and mice IgG receptors differ in their structures and aa sequences but also in their cellular expression pattern and their affinities towards the different IgG subtypes. Listing the discrepancies and similarities between murine and human IgG receptors would be beyond the scope of this review and was the topic of a recent one [31].
In summary, mIgG2a and mIgG2b can bind FcγRI, FcγRIIB, FcγRIII and FcγRIV and activate the complement through the classical pathway; mIgG3 does not bind any of the surface FcRs and is a minor complement activator; and finally, mIgG1 does not activate complement and binds weakly to FcγRIIB and FcγRIII (Table 1).
IgGs IN CRYOGLOBULINAEMIA
Clinical observations
As stated in the introduction, cryoglobulins are characterized by their property of precipitating when the temperature is under 37°C. This property explains the clinical manifestations observed in the distal extremities. However, this mechanism cannot explain cryoglobulinaemic glomerulonephritis since the kidney temperature does not drop under 37°C. However, the ultrafiltration taking place in glomeruli leads to an increase in blood protein concentration and formation of more important cryoglobulin complexes prone to precipitate inside and occlude the glomerular capillary loops.
IgGs’ functions are mediated through the activation of complement or receptors. The role of complement in cryoglobulinaemic vasculitis is suggested by numerous clinical observations and reduction of serum C4 has been included in a classification criteria questionnaire for the diagnosis of cryoglobulinaemic vasculitis [32, 33]. This is further strengthened by the correlation of low complement levels and renal involvement, as well as more abundant cryoglobulins, in HCV-related Type II cryoglobulinaemia [34]. More importantly, C3 deposition is a quasi-constant feature of renal pathology in cryoglobulinaemic glomerulonephritis [35]. However, treatment with eculizumab, an anti-C5 humanized antibody, inhibiting both the membrane attack complex and the C5a anaphylatoxin, of a woman with plasmapheresis-dependent cryoglobulinaemic glomerulonephritis failed to improve her renal status [36].
Surprisingly, in spite of the extended studies over Ig classes in cryoglobulinaemia [37], systematic studies of subclasses are few and their small studied populations do not afford one to reach any conclusion on potential IgG4 cryoglobulins [38, 39]. A similar observation regarding studies about the Fcγ receptor in cryoglobulinaemia can be made.
If the mechanism of cryoglobulins' pathogenicity is still unknown, a particular glycosylation pattern has been advanced as a reason for this pathogenicity. Indeed hyposialylation of an IgG may confer upon it cryoglobulin properties [40], a really interesting hypothesis in light of the influence of glycosylation on the affinity of IgGs to their receptors [41].
Mouse models
Cryoglobulinaemia can be easily induced in mice. Indeed, murine IgG3 are able to self-aggregate and cryoprecipitate through non-specific Fc–Fc interactions. Studies of mouse monoclonal IgG3 with cryoglobulin activity suggest that it could result from abnormal glycosylation, such as hypogalactosylation [42, 43] or hyposialylation [44, 45].
The role of complement in mouse cryoglobulinaemia is still not totally resolved. Indeed, the alternative pathway and, more precisely, anaphylatoxin generation have been reported as necessary for the development of cryoglobulinaemic glomerulonephritis [46], but recent studies reached contradicting results [5].
Conversely, studies about the role of FcγRs in murine cryoglobulinaemia reached the conclusion that activating receptors have no role in the aggravation of cryoglobulinaemic vasculitis [47]. However, macrophage activation seems to enhance the renal lesion, meaning that this activation may occur through ways other than IgG receptors [48].
Curiously, renal involvement and skin lesions seem to result from a distinct mechanism in mouse cryoglobulinaemia. Indeed, renal involvement can be seen in both Type I and mixed cryoglobulinaemias in mouse, whereas skin lesions need a component with rheumatoid factor activity [49]. However, in human cryoglobulinaemia, both skin lesions and renal involvement are as frequent in Type I as in mixed non-HCV-related cryoglobulinaemia [3, 50].
The protective role of mIgG1 in a mouse model
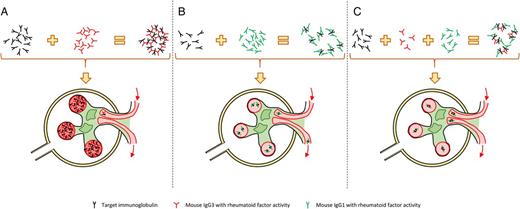
The mouse model of cryoglobulinaemia and cryoglobulinaemic vasculitis comprised auto-immune disease-prone mice [51], mice injected with hybridomas derived from MRL-lpr/lpr B-cells secreting mIgG3 with rheumatoid factor activity (anti-mIgG2a) [52] or transgenic mice overexpressing thymic stromal lymphopoietin [53]. In their recent paper, Strait et al. [5] described a new cryoglobulinaemia mouse model by injecting goat anti-mouse IgD antiserum (GaMD) to mice deficient in mIgG1 HC (γ1− mice). Indeed, the injection of GaMD elicited an anti-goat IgG humoral response that was predominantly mIgG1 and without any significant consequence in wild-type mice, whereas cryoglobulinaemia and cryoglobulinaemic vasculitis developed in γ1− mice in which mIgG3 was the principal constituent of the immune response (Figure 4A and B). Surprisingly, neither complement C3 nor common γ chain inactivations were able to reverse the mice phenotype, suggesting that the pathogenicity of antibodies elicited in the γ1− mice humoral response did not require their effector functions (via complement or FcRs). This result suggests that immune complexes may precipitate and induce renal lesions without any involvement of classical IgG effector functions.
Mechanisms of glomerular lesions in a cryoglobulinaemic glomerulonephritis model and their prevention. (A) Mouse IgG3 (mIgG3) have flexible arms that allow them to bind two antigens and form high weight immune complexes. These complexes block glomerular capillaries, induce vascular plotting and alteration of glomerular function. (B) Mouse IgG1 (mIgG1) are less flexible than mIgG3, inducing smaller immune complexes unable to obstruct the glomerular vessels. (C) Adding mIgG1 to mIgG3 with specificity towards the same antigen leads to the formation of intermediate size immune complexes small enough to pass through the glomerular tuft without causing renal lesions.
The second conclusion suggested by these experiments is a protective role of mIgG1 itself, which was confirmed by the rescue of the renal phenotypes by injection of serum from the GaMD-immunized wild-type mice. Through another series of experiments, they were able to further explain that this protective effect could only be effective when both mIgG1 and mIgG3 recognized the same antigen (Figure 4C). This was also supported by the fact that FcγR−/γ1+ mice did not develop cryoglobulinaemia and FcγRIIb/γ1− mice did, meaning that the IgG1 protective role was independent of ITIM or inhibitory ITAM. This mIgG1 protective role may result from the lesser mIgG1 hinge region flexibility compared with that of mIgG3. Indeed, this difference between mIgG1 and mIgG3 may be responsible for their difference in C1q binding. Moreover, as mIgG1 and hIgG4 hinge region flexibilities are pretty similar [29], the authors suggest that hIgG4 may be even less prone to cross-link antigens and form immune complexes since, in addition to its short hinge region, one hIgG4 molecule may be bispecific through its arm exchange property [7]. The functional equivalence of mIgG1 and hIgG4 is further supported by the requirement of IL-4 to induce mIgG1 [54] and hIgG4 [55]. However, it has been found that Ig may exert immunomodulatory functions through other receptors such as FcRL4, FcRL5 [24] and DC-SIGN (SIGN-R1 in mice) [12]. Although FcRL5 is expressed in B-cells, which makes their involvement in the mIgG1 protective role unlikely, SIGN-R1 is expressed by macrophages and its role cannot be ruled out (Table 2).
Summary
| • Humans possess five IgG subtypes that differ in the structure of their hinge region and their disulphide bonds. |
| • IgG glycosylation consists of a conserved core glycan through all subtypes but different ramifications may influence IgG functions. |
| • IgG functions require binding to several effector molecules (C1q and receptors) whose affinities towards the different IgG subtypes are not equivalent, explaining the unequal functions of IgG subtypes. |
| • Mouse and human IgGs share similarities but also show discrepancies. |
| • In spite of the low serum complement in patients with cryoglobulinaemic vasculitis, a role of complement has never been demonstrated in the mouse model. |
| • The unequivalent ability of mouse IgG subclasses to exhibit cryoglobulin properties is not explained by their affinity to Fc receptor or their ability to activate complement, but rather by the flexibility of their hinge region. |
| • Humans possess five IgG subtypes that differ in the structure of their hinge region and their disulphide bonds. |
| • IgG glycosylation consists of a conserved core glycan through all subtypes but different ramifications may influence IgG functions. |
| • IgG functions require binding to several effector molecules (C1q and receptors) whose affinities towards the different IgG subtypes are not equivalent, explaining the unequal functions of IgG subtypes. |
| • Mouse and human IgGs share similarities but also show discrepancies. |
| • In spite of the low serum complement in patients with cryoglobulinaemic vasculitis, a role of complement has never been demonstrated in the mouse model. |
| • The unequivalent ability of mouse IgG subclasses to exhibit cryoglobulin properties is not explained by their affinity to Fc receptor or their ability to activate complement, but rather by the flexibility of their hinge region. |
Summary
| • Humans possess five IgG subtypes that differ in the structure of their hinge region and their disulphide bonds. |
| • IgG glycosylation consists of a conserved core glycan through all subtypes but different ramifications may influence IgG functions. |
| • IgG functions require binding to several effector molecules (C1q and receptors) whose affinities towards the different IgG subtypes are not equivalent, explaining the unequal functions of IgG subtypes. |
| • Mouse and human IgGs share similarities but also show discrepancies. |
| • In spite of the low serum complement in patients with cryoglobulinaemic vasculitis, a role of complement has never been demonstrated in the mouse model. |
| • The unequivalent ability of mouse IgG subclasses to exhibit cryoglobulin properties is not explained by their affinity to Fc receptor or their ability to activate complement, but rather by the flexibility of their hinge region. |
| • Humans possess five IgG subtypes that differ in the structure of their hinge region and their disulphide bonds. |
| • IgG glycosylation consists of a conserved core glycan through all subtypes but different ramifications may influence IgG functions. |
| • IgG functions require binding to several effector molecules (C1q and receptors) whose affinities towards the different IgG subtypes are not equivalent, explaining the unequal functions of IgG subtypes. |
| • Mouse and human IgGs share similarities but also show discrepancies. |
| • In spite of the low serum complement in patients with cryoglobulinaemic vasculitis, a role of complement has never been demonstrated in the mouse model. |
| • The unequivalent ability of mouse IgG subclasses to exhibit cryoglobulin properties is not explained by their affinity to Fc receptor or their ability to activate complement, but rather by the flexibility of their hinge region. |
SUMMARY AND FUTURE DIRECTIONS
IgGs are not homogeneous in terms of structures and functions. They exert their function through binding of effector molecules (C1q or membrane receptor). Recently, glycosylation of their CH2 domain near the hinge region has emerged as an important feature (reviewed in [56]) that may mediate and modulate IgG functions, an observation as valid in humans as in mouse in spite of important differences between both species. Strait et al. [5] recently unveiled a novel immunomodulatory mechanism of mouse IgG1 independent of conventional effector mechanisms but dependent on their biochemical and structural properties only. However, involvement of unconventional IgG receptors may not be ruled out. Indeed, the FcRn may be responsible for an increased half-life of mIgG3 with cryoglobulin activity in the absence of mIgG1. Moreover, SIGN-R1, a mouse C-type lectin receptor equivalent to the human DC-SIGN expressed by DCs and macrophages, binds to sialylated IgG and promotes an anti-inflammatory signal [12]. However, the authors did not study the glycosylation of mIgG1 and mIgG3 of their mice.
The authors pointed out some of the common characteristics of mIgG1 and hIgG4 and hypothesized that hIgG4 may have the same protective properties towards cryoglobulinaemia in humans as mIgG1 in their model. Their hypothesis would be supported if it were shown that patients with hIgG4 monoclonal gammopathy [38, 57] had less or no cryoglobulin compared with patients with hIgG1, hIgG2 or hIgG3 monoclonal gammopathy. Then, we may further imagine, should the preceding hypothesis turn out to be confirmed, that patients with mixed cryoglobulinaemia could benefit from infusion of monoclonal hIgG4 with rheumatoid factor activity.
CONFLICT OF INTEREST STATEMENT
None declared.





Comments