-
PDF
- Split View
-
Views
-
Cite
Cite
Sandra M.S. Herrmann, Stephen C. Textor, Diagnostic criteria for renovascular disease: where are we now?, Nephrology Dialysis Transplantation, Volume 27, Issue 7, July 2012, Pages 2657–2663, https://doi.org/10.1093/ndt/gfs254
Close - Share Icon Share
Abstract
Renovascular disease, especially atherosclerotic renal artery stenosis (ARAS) in older subjects, is commonly encountered in clinical practice. This is at least in part due to the major advances in non-invasive imaging techniques that allow greater diagnostic sensitivity and accuracy than ever before. Despite increased awareness of ARAS, renal revascularization is less commonly performed, likely as a result of several prospective, randomized, clinical trials which fail to demonstrate major benefits of renal revascularization beyond medical therapy alone. Primary care physicians are less likely to investigate renovascular disease and nephrologists likely see more patients after a period of unsuccessful medical therapy with more advanced ARAS. The goal of this review is to revisit current diagnostic and therapeutic paradigms in order to characterize more clearly which patients will likely benefit from further evaluation and intensive treatment of renal artery stenosis.
Introduction
The landscape of clinical renovascular disease (RVD) has shifted dramatically over the last decade. Major advances in non-invasive vascular imaging allow more frequent detection and precise diagnostic assessment than ever before. In the past, it was often considered automatic to propose revascularization for individuals with high-grade RVD [1]. However, results from several prospective, randomized clinical trials comparing current medical therapy with or without renal revascularization largely favor a conservative approach for patients with moderate vascular disease [2, 3]. Indeed, many patients have moderate, but radiologically evident, disease that remains clinically silent. On the other hand, experienced clinicians recognize that important subsets of patients with significant atherosclerotic renal artery stenosis (ARAS), refractory hypertension and progressive vascular injury may benefit enormously from renal revascularization. How to identify and select these patients optimally, however, remains challenging. The goal of this review is therefore to delineate this topic further and to characterize more clearly those patients with hypertension who likely will benefit from further diagnostic evaluation of renal artery stenosis. Our overall premise is that clinical evaluation of ARAS is a two-step process: (i) clinical determination that medical therapy alone is insufficient and that further studies with the intention of restoring the renal blood flow are warranted and (ii) establishing that hemodynamically significant ARAS is present and treatable.
Clinical criteria for pursuing the initial diagnosis of renovascular disease
Several clinical features raise the suspicion for RVD (Table 1) and should prompt further consideration [4]. These features include an early onset of hypertension below the age of 30 with a negative family history, late onset (above age 55) or accelerated and especially severe or resistant hypertension. Additional clinical features include asymmetric kidneys with >1.5 cm of difference in the size and/or otherwise unexplained loss of kidney function. More specific clinical findings are acute elevation of serum creatinine (>30% above pre-treatment levels) after initiation of renin–angiotensin system inhibitor. Realistically defining these features requires a willingness to advance antihypertensive drug therapy, evaluate medication adherence and clinical response over a period of time, sometimes for months. If acceptable blood pressure control can be achieved with an easily tolerated drug regimen and renal function remains stable, many would argue that little is to be gained by undertaking further expensive and occasionally hazardous imaging and vascular intervention [5, 6]. Conversely, if successful blood pressure control remains hard to achieve despite careful buildup of drug therapy, both patient and physician can better accept the potential benefits of identifying reversible components underlying elevated systemic pressures.
Adapted from 2005 ACC/AHA practice guidelines
| Clinical findings associated with renovascular disease |
| Onset hypertension before age of 30 years old |
| Accelerated, resistant, malignant hypertension |
| Deterioration of renal function in response to angiotensin-converting enzyme inhibitors or angiotensin-receptor blocker |
| New onset of hypertension after 50 years of age (suggestive of atherosclerotic renal artery stenosis) |
| Asymmetric kidneys with more than 1.5 cm of difference in the size and otherwise unexplained loss of kidney function |
| Sudden unexplained pulmonary edema |
| Clinical findings associated with renovascular disease |
| Onset hypertension before age of 30 years old |
| Accelerated, resistant, malignant hypertension |
| Deterioration of renal function in response to angiotensin-converting enzyme inhibitors or angiotensin-receptor blocker |
| New onset of hypertension after 50 years of age (suggestive of atherosclerotic renal artery stenosis) |
| Asymmetric kidneys with more than 1.5 cm of difference in the size and otherwise unexplained loss of kidney function |
| Sudden unexplained pulmonary edema |
Adapted from 2005 ACC/AHA practice guidelines
| Clinical findings associated with renovascular disease |
| Onset hypertension before age of 30 years old |
| Accelerated, resistant, malignant hypertension |
| Deterioration of renal function in response to angiotensin-converting enzyme inhibitors or angiotensin-receptor blocker |
| New onset of hypertension after 50 years of age (suggestive of atherosclerotic renal artery stenosis) |
| Asymmetric kidneys with more than 1.5 cm of difference in the size and otherwise unexplained loss of kidney function |
| Sudden unexplained pulmonary edema |
| Clinical findings associated with renovascular disease |
| Onset hypertension before age of 30 years old |
| Accelerated, resistant, malignant hypertension |
| Deterioration of renal function in response to angiotensin-converting enzyme inhibitors or angiotensin-receptor blocker |
| New onset of hypertension after 50 years of age (suggestive of atherosclerotic renal artery stenosis) |
| Asymmetric kidneys with more than 1.5 cm of difference in the size and otherwise unexplained loss of kidney function |
| Sudden unexplained pulmonary edema |
An important additional constellation of conditions characterized by recurrent, refractory congestive heart failure, sudden ‘flash’ pulmonary edema or unstable angina as a result of rapidly rising arterial pressure rather than a true acute coronary syndrome have been designated as the ‘cardiorenal’ syndromes. Symptoms of rapidly developing pulmonary edema reflect defective natriuresis often associated with bilateral RVD, magnified by diastolic dysfunction of the left ventricle associated with a rapid rise in blood pressure and failure of the pulmonary capillary blood-gas exchange barrier as reviewed recently [7].
Regardless of the specific clinical context, extending the work-up to further imaging and other diagnostic procedures implies sufficient clinical commitment to adjust therapy based on the results. We believe it to be prudent to emphasize this to the patient and all concerned beforehand.
Diagnostic criteria for RVD: Doppler, computed tomography angiography and magnetic resonance angiography
Advanced non-invasive imaging modalities have become focal points in the evaluation of ARAS [8, 9]. The choice of initial imaging depends both on patient characteristics, local availability and expertise. Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) are more costly compared with Doppler ultrasound and usually require contrast exposure. However, none of these tests completely excludes the disease if negative, and they are most informative for clinical purposes when the results are positive in a population at high risk for disease. In some instances, further CT or MR studies may be warranted after a Doppler study to better define vascular anatomy, renal functional characteristics and anomalies before proceeding with intra-arterial angiography. Defining the specific goals of diagnostic imaging in advance will increase the value of these procedures [10]. In some cases, the primary goal may be simply to identify if high-grade stenosis is present at all. In others, it may be used to determine whether lesions are bilateral, affect all vessels and/or are in location suitable for either endovascular or surgical repair. The value and sequence of imaging may vary depending upon the specific goals for each patient. If clinical suspicion for severe ARAS is high and non-invasive tests remain inconclusive, catheter angiography may be performed, although this procedure now is typically reserved for patients considered candidates for endovascular intervention at the same session.
Duplex Doppler renal ultrasonography is an excellent initial imaging tool and can provide both functional and structural assessment [11]. Because of its limited expense, ultrasound can be used to follow patients serially and to evaluate vascular patency after revascularization. The peak systolic velocity (PSV) has the highest performance characteristics and reaches a sensitivity of 85% and a specificity of 92% for the diagnosis of ARAS [12]. The limitations of this technique hinge upon its dependence upon operator skills and patient body habitus, leading to reported accuracy estimates that range from 60 to >90% [9]. The resistive index (RI) is determined from segmental arterial flow characteristics. The RI is defined as height of the peak systolic velocity (PSV) minus height of the end-diastolic velocity (EDV) divided by the PSV [RI = (PSV – EDV)/PSV] and thus reflects the status of the flow characteristics in the renal microcirculation beyond the main renal arteries. An elevated RI indicates limited diastolic flow and may reflect intrinsic parenchymal or small-vessel disease. In conjunction with clinical findings, RI has been promoted as a useful parameter to predict benefit after revascularization [13, 14]. Radermacher et al. [13, 15, 16] report that patients with RI >0.8 before angioplasty have worse renal outcomes compared with those with an RI <0.8. Zeller et al. [16] evaluated a cohort of 241 patients treated with endovascular stenting with severe ARAS (>70%) and found that 39 of the 241 patients had RI >0.8 and nonetheless did have clinical improvement of blood pressure and renal function at 6 months after revascularization. In contrast, Garcia-Criado et al. [15] report similar renal outcomes for patients with RI >0.8 and those with RI <0.8 in a cohort of 36 revascularized patients (Table 2). Hence, reliance upon RI as a predictive parameter for ARAS management remains controversial. Our interpretation of these studies is that lower RI likely is associated with more preserved renal flow characteristics and better kidney function overall, but should not determine the final decision for revascularization.
Blood pressure and renal function response after revascularization based on resistive index by duplex ultrasound
| Reference . | Definition of outcome . | RI . | BP outcome . | RF outcome . |
|---|---|---|---|---|
| Santos et al. [14] | No benefit (NB), improvement (I) or cure (C) of BP | >0.8 | 5% improvement | No difference of RF in either group |
| <0.8 | 70% improvement 2.5% cure | |||
| Radermacher et al. [13] | Mean arterial BP: ↓ by 10% after RV | >0.8 | 3% improvement | 80% RF worsening |
| RF: worsening of CrCl by 10% after RV | <0.8 | 94% improvement | 3% RF worsening | |
| Zeller et al. [16] | Mean arterial BP: ↓ by 5 mmHg after RV | >0.8 | Improvement of BP is similar in all groups | Improvement of Scr is similar in all groups |
| RF:↓ Scr by 10% at 6 months after RV | 0.7–0.8 | |||
| <0.8 | ||||
| Garcia-Criado et al. [15] | Diastolic BP: ↓ by 15% after RV | >0.8 | 50% improvement | 29% improvement |
| RF:↓ Scr by 20% after RV | <0.8 | 85% improvement | 45% improvement |
| Reference . | Definition of outcome . | RI . | BP outcome . | RF outcome . |
|---|---|---|---|---|
| Santos et al. [14] | No benefit (NB), improvement (I) or cure (C) of BP | >0.8 | 5% improvement | No difference of RF in either group |
| <0.8 | 70% improvement 2.5% cure | |||
| Radermacher et al. [13] | Mean arterial BP: ↓ by 10% after RV | >0.8 | 3% improvement | 80% RF worsening |
| RF: worsening of CrCl by 10% after RV | <0.8 | 94% improvement | 3% RF worsening | |
| Zeller et al. [16] | Mean arterial BP: ↓ by 5 mmHg after RV | >0.8 | Improvement of BP is similar in all groups | Improvement of Scr is similar in all groups |
| RF:↓ Scr by 10% at 6 months after RV | 0.7–0.8 | |||
| <0.8 | ||||
| Garcia-Criado et al. [15] | Diastolic BP: ↓ by 15% after RV | >0.8 | 50% improvement | 29% improvement |
| RF:↓ Scr by 20% after RV | <0.8 | 85% improvement | 45% improvement |
RI, resistive index; %, percentage; BP, blood pressure; mmHg, millimeters of mercury; RF, renal function; RV, revascularization; Scr, serum creatinine; ↓, decrease; CrCL, creatinine clearance.
Blood pressure and renal function response after revascularization based on resistive index by duplex ultrasound
| Reference . | Definition of outcome . | RI . | BP outcome . | RF outcome . |
|---|---|---|---|---|
| Santos et al. [14] | No benefit (NB), improvement (I) or cure (C) of BP | >0.8 | 5% improvement | No difference of RF in either group |
| <0.8 | 70% improvement 2.5% cure | |||
| Radermacher et al. [13] | Mean arterial BP: ↓ by 10% after RV | >0.8 | 3% improvement | 80% RF worsening |
| RF: worsening of CrCl by 10% after RV | <0.8 | 94% improvement | 3% RF worsening | |
| Zeller et al. [16] | Mean arterial BP: ↓ by 5 mmHg after RV | >0.8 | Improvement of BP is similar in all groups | Improvement of Scr is similar in all groups |
| RF:↓ Scr by 10% at 6 months after RV | 0.7–0.8 | |||
| <0.8 | ||||
| Garcia-Criado et al. [15] | Diastolic BP: ↓ by 15% after RV | >0.8 | 50% improvement | 29% improvement |
| RF:↓ Scr by 20% after RV | <0.8 | 85% improvement | 45% improvement |
| Reference . | Definition of outcome . | RI . | BP outcome . | RF outcome . |
|---|---|---|---|---|
| Santos et al. [14] | No benefit (NB), improvement (I) or cure (C) of BP | >0.8 | 5% improvement | No difference of RF in either group |
| <0.8 | 70% improvement 2.5% cure | |||
| Radermacher et al. [13] | Mean arterial BP: ↓ by 10% after RV | >0.8 | 3% improvement | 80% RF worsening |
| RF: worsening of CrCl by 10% after RV | <0.8 | 94% improvement | 3% RF worsening | |
| Zeller et al. [16] | Mean arterial BP: ↓ by 5 mmHg after RV | >0.8 | Improvement of BP is similar in all groups | Improvement of Scr is similar in all groups |
| RF:↓ Scr by 10% at 6 months after RV | 0.7–0.8 | |||
| <0.8 | ||||
| Garcia-Criado et al. [15] | Diastolic BP: ↓ by 15% after RV | >0.8 | 50% improvement | 29% improvement |
| RF:↓ Scr by 20% after RV | <0.8 | 85% improvement | 45% improvement |
RI, resistive index; %, percentage; BP, blood pressure; mmHg, millimeters of mercury; RF, renal function; RV, revascularization; Scr, serum creatinine; ↓, decrease; CrCL, creatinine clearance.
What constitutes ‘significant’ renal arterial disease?
The answer to this question depends more upon the hemodynamic consequences of a lesion than its anatomic appearance. Traditionally, ‘significant’ stenosis has been defined by an approximate renal artery cross-sectional occlusion of at least 50% [17]. However, studies using latex casts to characterize luminal occlusion fail to detect measurable pressure or flow changes at levels <60% and indicate that the threshold for hemodynamic effects develops between 75 and 85% of luminal occlusion [18]. Doppler studies indicate that a luminal stenosis of 60% is associated with PSV of 200 cm/s [19]. Many clinical reports suggest that non-invasive estimates of stenosis often exaggerate the degree of occlusion. A substantial number of patients enrolled in the STAR trial assigned to renal artery stenting, for example, were not treated because actual stenoses at the time of angiography were trivial [2]. As a corollary, recent studies evaluating duplex velocities with quantitative angiography suggest that 60% stenoses are better detected with velocities >300 cm/s [20]. Our own studies utilizing blood oxygen level-dependent (BOLD) MR to identify cortical hypoxia in severe renal arterial disease suggest that velocities >385 cm/s are more commonly associated with renal hypoxia [21].
Activation of renal pressor systems, most notably the renin–angiotensin–aldosterone system (RAAS) depends on sufficient lumen reduction to reduce post-stenotic renal perfusion pressure. Studies in humans using expanded balloon occlusion show that renin release does not occur until the pressure distal to the lesion falls at least by 10–20% below the pressure proximal to the lesion [22]. This corresponds to translesional peak systolic gradients of 20–25 mmHg and degrees of luminal stenoses of 70–80% [23]. These data are consistent with hyperemic studies using dopamine to induced translesional mean pressure gradients. These results indicate that gradients >20 mmHg independently predict improvement of hypertension after revascularization [24].
Advances in imaging technology favor expanded use of spiral multidetector CT angiography and MRA as valid methods to visualize ARAS [25]. Compared with catheter-based renal angiography, these modalities are less invasive, allow multi-planar imaging of the arteries and soft tissue and are suitable for complex reconstruction analysis [26]. CTA and MRA are of comparable accuracy, reaching sensitivity and specificity >90% in a number of single-center studies compared with catheter angiography [25]. Leung et al. [27] used breath-hold contrast enhanced MRA in 96 renovascular patients without fibromuscular dysplasia (FMD) and found that MRA had a sensitivity of 97% and negative predictive value of 98% for the detection of renal artery stenoses of at least 60%. Eklof et al. [28] and Rountas et al. [29] also compared accuracy of CTA and MRA to catheter angiography in 58 renovascular patients, showing sensitivity >90% for both modalities. However, specificity was lower (62%) in Eklof's study. The authors relate the discrepancy in the results to borderline lesions (Table 3). Even though CTA offers better spatial resolution currently, MRA has the advantage of avoiding radiation. The main limitations of these imaging studies include the risk of contrast nephropathy with CTA and concerns regarding the potential for nephrogenic systemic fibrosis in patients with significant renal insufficiency [glomerular filtration rate (GFR) <30 mL/min/1.73 m2] receiving gadolinium with MRA [30].
Sensitivity and specificity of CTA and MRA on diagnosis renal artery stenosis
| Reference . | Modality . | Year . | No. of patients . | Stenosis (%) . | Sensitivity (%) . | Specificity (%) . |
|---|---|---|---|---|---|---|
| Leung et al. [27] | MRA | 1999 | 89 | 60 | 97 | 85 |
| Eklof et al. [28] | CTA | 2006 | 58 | 50 | 94 | 62 |
| MRA | 93 | 91 | ||||
| Rountas et al. [29] | CTA | 2007 | 58 | 50–99 | 94 | 93 |
| MRA | 90 | 94 |
| Reference . | Modality . | Year . | No. of patients . | Stenosis (%) . | Sensitivity (%) . | Specificity (%) . |
|---|---|---|---|---|---|---|
| Leung et al. [27] | MRA | 1999 | 89 | 60 | 97 | 85 |
| Eklof et al. [28] | CTA | 2006 | 58 | 50 | 94 | 62 |
| MRA | 93 | 91 | ||||
| Rountas et al. [29] | CTA | 2007 | 58 | 50–99 | 94 | 93 |
| MRA | 90 | 94 |
CTA, computed tomography angiography; MRA, magnetic resonance angiography; %, percentage.
Sensitivity and specificity of CTA and MRA on diagnosis renal artery stenosis
| Reference . | Modality . | Year . | No. of patients . | Stenosis (%) . | Sensitivity (%) . | Specificity (%) . |
|---|---|---|---|---|---|---|
| Leung et al. [27] | MRA | 1999 | 89 | 60 | 97 | 85 |
| Eklof et al. [28] | CTA | 2006 | 58 | 50 | 94 | 62 |
| MRA | 93 | 91 | ||||
| Rountas et al. [29] | CTA | 2007 | 58 | 50–99 | 94 | 93 |
| MRA | 90 | 94 |
| Reference . | Modality . | Year . | No. of patients . | Stenosis (%) . | Sensitivity (%) . | Specificity (%) . |
|---|---|---|---|---|---|---|
| Leung et al. [27] | MRA | 1999 | 89 | 60 | 97 | 85 |
| Eklof et al. [28] | CTA | 2006 | 58 | 50 | 94 | 62 |
| MRA | 93 | 91 | ||||
| Rountas et al. [29] | CTA | 2007 | 58 | 50–99 | 94 | 93 |
| MRA | 90 | 94 |
CTA, computed tomography angiography; MRA, magnetic resonance angiography; %, percentage.
Should clinicians use functional tests for renovascular disease?
Using captopril, radionucleotide renography has been used to evaluate RVD. Diethylenetriaminepentaacetic acid and mercapto-acetyltriglycine (MAG 3) are the most commonly employed radionucleotides with the latter being more reliable in renal insufficiency, since MAG3 is secreted effectively by the proximal tubule [31].The criteria for RVD include (i) a decrease in the percentage of uptake of the isotope by the affected kidney to <40% of the total, (ii) delayed time to peak uptake of the isotope to >10–11 min, well above the normal value of 6 min and (iii) delayed excretion of the isotope with retention at 25 min or >20%. The addition of captopril and comparison with a baseline (non-captopril) renogram allow estimation of the functional role of angiotensin in maintaining glomerular filtration and exaggerates hemodynamic differences between a kidney with stenosis and one without. In a selected population of patients with known ARAS, renograms before and after captopril are highly sensitive (83%) and specific (93%) in detecting unilateral renal artery stenosis in patients with normal renal function and >70% stenosis [32]. However, this test does not distinguish reliably unilateral and bilateral ARAS. Among patients with bilateral disease, asymmetry was identified in the more severely affected kidney, but the presence or absence of stenosis in the contralateral kidney could not be reliably identified [33]. Most importantly, renogram sensitivity and specificity decrease with decline of renal function, especially for patients who have serum creatinine levels >2 mg/dL. As summarized in a recent meta-analysis, renogram sensitivity ranges from 58 to 95% and its specificity ranges from 17 to 100%, even when studies were performed in selected patients who had an intervention based on positive results on angiography [25]. The role of renography in the current era may be primarily to evaluate the relative function of each kidney prior to proceeding with therapeutic nephrectomy.
Pressor systems in renovascular hypertension: plasma renin activity
Renovascular occlusive disease, regardless of the cause, activates multiple pressor systems, most notably the RAAS. Experimental models using the 2- or 1-kidney-1-clip hypertension are among the most extensively studied secondary forms of hypertension and remain a prototype of ‘angiotensin-dependent’ hypertension [34]. Initial activation of the RAAS is necessary for these models, but is often transient. Later in the course of these disorders additional pathways are recruited which include oxidative stress injury, sympathoadrenergic activation and impaired vasodilatation [35]. Not surprisingly, numerous studies have attempted to utilize measurements of the plasma renin activity (PRA) to identify renovascular hypertension and to predict blood pressure response to renal revascularization. Peripheral PRA has been disappointing in clinical use. It is not sensitive enough under routine conditions to reliably diagnose renovascular hypertension. It is elevated in only half of the patients and is variably affected by ethnicity, age, medications, volume status and other variables [36].
PRA measurements performed from renal venous sampling have been widely used to plan surgical renal revascularization in the past and have a better positive predictive value for the response to therapy [37]. Indeed, a ratio of renal vein renin levels >1.5 has a predictive positive value for blood pressure improvement up to 92% in some studies [38]. Some authors propose that a ‘net contribution’ of PRA from the stenotic kidney (defined as 100 * [(renal vein PRA − infrarenal vena cava (IVC) PRA)/IVC PRA]) >24% indicates excessive production of renin and levels >48% meet Vaughan criteria for curability in patients with unilateral disease [39, 40]. Conditions for testing are critical; however, the degree of lateralization is sensitive to volume status, concurrent medications and arterial pressure levels. An additional limitation of renal vein measurements is the invasive nature of the procedure. Although widely used to identify candidates for surgical revascularization, use of renal vein renin measurements fell off during the era of expanding endovascular angioplasty and stenting. In view of ambiguous results from recent endovascular trials, some may argue that careful patient selection may benefit from revisiting this diagnostic approach.
Estimating the benefits of revascularization: when is it too late?
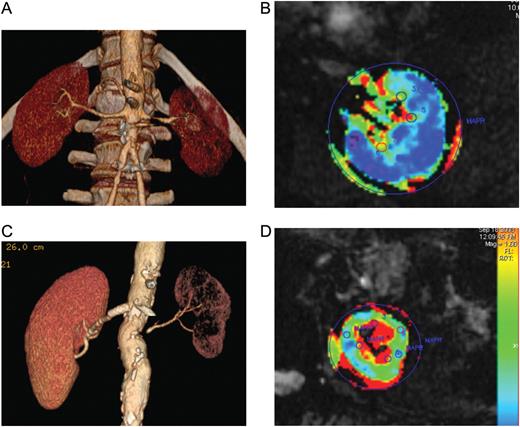
Although catheter-based techniques have improved and complication rates have decreased over the past two decades, the clinical benefits of renal revascularization remain unclear. Two trials examining the role of revascularization to slow progression of kidney disease in ARAS identify no added benefit from endovascular stenting. Recent studies of renal adaptation to ARAS provide one possible explanation by demonstrating remarkable preservation of oxygenation despite substantial reductions in the blood flow and GFR in post-stenotic kidneys. Gloviczki et al. [41] demonstrated that stenotic kidneys of patients with unilateral RVD have reduced the blood flow and GFR compared with patients with essential hypertension. Tissue oxygenation as defined by R2* values (a measure of deoxyhemoglobin) using BOLD MR are preserved in cortical and medullary regions for most of these patients despite of the presence of high-grade lesions (mean 71%) [42]. These data argue that ‘adaptation’ of the post-stenotic kidney allows a reduction in GFR without worsening overt tissue hypoxia. Hence, many ARAS patients tolerate antihypertensive drug therapy with reduced kidney function without necessarily being subjected to further tissue injury. These observations are fully consistent with the observations of reasonably stable renal function for many patients treated medically in previous series and in the prospective trials [2, 3, 43]. Optimizing antihypertensive drug therapy before deciding upon the need for further evaluation usually poses little hazard over the short term (Figure 1).
CT angiography and blood oxygen level-dependent (BOLD) MR: (A and C) CT angiograms depicting unilateral moderate (A) and severe (B) atherosclerotic renal artery stenosis affecting the left kidney in each case. Both were treated with antihypertensive drug therapy including ACE/ARB inhibition before evaluation. In case (A), BOLD imaging in an axial cross-sectional image at 3 T demonstrates low levels of deoxyhemoglobin in the cortex (blue areas) with a normal gradient to higher levels of deoxyhemoglobin (yellow-red zones in the medulla). In this patient, reduction in the blood flow does not produce overt hypoxia in the kidney itself (see text.). In contrast, the individual in Panel (C) has more severe and advanced vascular occlusion to the smaller kidney associated with evident cortical hypoxia (green zone in the cortex) and widening fraction of the medulla with overt hypoxia (red zone). These measurements allow the identification of kidney tissue for which ARAS poses little risk of progressive renal injury (A and B) when compared with a kidney with advancing occlusive injury, hypoxia and ischemic nephropathy (C and D).
It is equally clear, however, that high-grade RVD ultimately reaches a limit to these ‘adaptive’ mechanisms. Analysis of tissue oxygenation using BOLD MR for kidneys with more severe renovascular stenosis (mean 77%), defined by Doppler velocities >385 cm/s and advancing loss of GFR, did identify loss of tissue perfusion and cortical oxygenation compared with a less severe RVD (higher cortical R2* level, i.e. 22 versus 16) [42]. Biopsy samples from advanced disease demonstrate progressive accumulation of inflammatory cells and interstitial fibrosis [44]. AT1 receptor activation of T cells is capable of activating sympathoadrenergic and chemokine pathways leading to vasoconstriction and inflammation [45]. Murine 2-kidney-1-clip hypertension models are characterized by stimulation of TGF-β and recruitment of other inflammatory cells as a consequence of the kidney injury [46]. Studies in a swine model indicate that the ‘atherosclerotic milieu’ itself further modifies vascular reactivity and accelerates rarefaction of renal microvessels [47]. The atherosclerotic environment and age associated with ARAS may explain part of the relative infrequency of tissue injury in subjects with FMD when compared with atherosclerotic disease [35]. These data underscore the need for preservation of the renal microcirculation. Important predictors of the functional renal outcome in clinical ARAS are the degree of histopathological damage and the time of duration of RVD [48]. Taken together, nephrologists must recognize that reduced renal perfusion ultimately can reach a point beyond which the kidney suffers tissue injury, which may be irreversible. These considerations underscore the urgent need for better tools to identify kidneys at risk for injury that may benefit from revascularization and other reparative procedures.
When to rely on medical therapy—and when not to
Intensive management of atherosclerotic disease remains a cornerstone for treating all patients with ARAS, both before and after revascularization. Objectives in the management of RVD include optimizing blood pressure control, preservation of renal function and prevention of complications such as recurrent flash pulmonary edema. Beyond lifestyle modification and antihypertensive drug therapy, blockade of the RAAS is important for those who are able to tolerate it. Both clinical series and Canadian pharmacy registration data indicate that the use of ACE inhibitors or ARBs offers survival advantages for patients with ARAS [49]. Up-regulation of angiotensin II has been linked to inflammation and oxidative stress [50]. Furthermore, beta-blockade often is recommended in this population due to the increased cardiovascular risk. Statin therapy is also important based on concomitant cardiovascular disease and may slow rates of deterioration of renal function [51, 52].
It should be self-evident that failure to achieve adequate blood pressure control and/or stable kidney function should prompt re-examination of this issue. Specific outcomes and hazards associated with renal revascularization are beyond the scope of this review. Nonetheless, successful revascularization of pressor kidneys undoubtedly lowers antihypertensive drug requirements [53]. Previous studies indicate that a short duration of renovascular hypertension is among the strongest predictors of clinical benefit of renovascular intervention [35]. However, a recent meta-analysis could identify no specific clinical characteristic that reliably predicts the renal functional outcome. The best clinical predictor was larger diastolic blood pressure (DBP) reduction in patients with high pretreatment DBP [54].
One predictable result of recent negative trials (ASTRAL, STAR) is that primary physicians are less likely to pursue the diagnosis of ARAS. We are already seeing patients that have been managed medically now presenting with more advanced ARAS than before. More than ever, we believe that the role of the nephrologist necessarily will focus on recognizing patients with ARAS in time to both evaluate their severity and to identify those patients who may benefit from vascular intervention.
Acknowledgement
The project described was supported by Award Number PO1HL85307 from the National Heart, Lung and Blood Institute and NIH/NCRR CTSA Grant Number UL1 RR024150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.





Comments